Nature Cell Challenges MFDS Criteria as FDA Pathway Gains Momentum
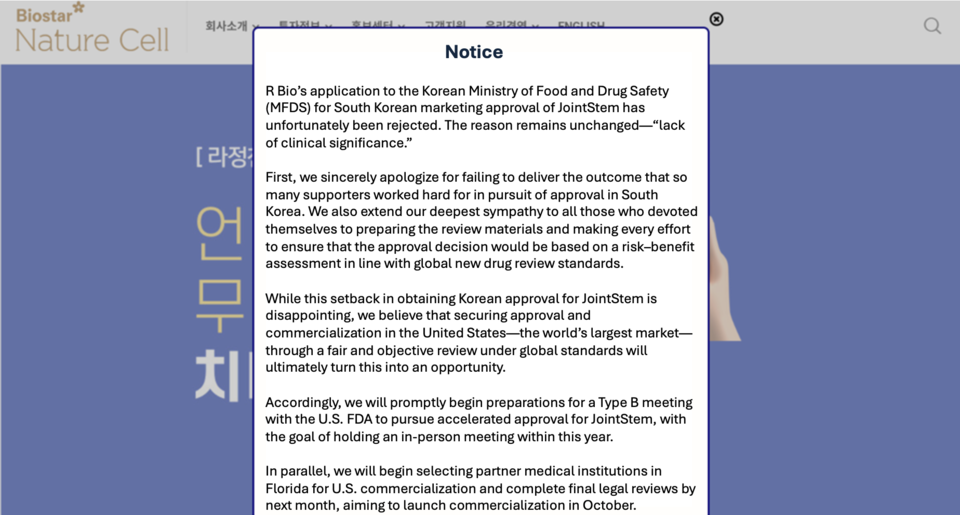
Nature Cell’s degenerative arthritis stem cell therapy, JointStem, has been denied South Korean marketing approval for the second time, reigniting debate over how the Ministry of Food and Drug Safety (MFDS) defines “clinical significance.” The rejection, identical in reasoning to the agency’s 2021 decision, comes despite additional long-term data and U.S. regulatory designations.
On August 6, Nature Cell announced via its website that R Bio, the Korean license holder, had its application rejected following review by the Central Pharmaceutical Affairs Council on June 23. JointStem—an autologous adipose-derived mesenchymal stem cell therapy administered as a single intra-articular injection—was first refused conditional approval in 2018, then formally denied in 2023 for showing statistical but not clinical significance in Phase 3 trials.
To address that finding, R Bio resubmitted in March 2024 with three-year follow-up data and real-world evidence, including reduced knee replacement rates. In parallel, the U.S. FDA granted 카지노 바카라 both Regenerative Medicine Advanced Therapy (RMAT) and Breakthrough Therapy (BT) status in 2024, raising expectations for a favorable Korean review.

Ongoing Dispute Over ‘Clinical Significance’ Standards
What does “lack of clinical significance” mean?
Statistical significance shows that trial results are unlikely due to chance, while clinical significance measures whether those results translate into meaningful patient benefit. In JointStem’s case, Phase 3 WOMAC and VAS scores met statistical thresholds, but the MFDS determined they fell short of pre-set trial expectations—benchmarks never disclosed as formal approval criteria. Critics argue the assessment relied on subjective judgment rather than standardized measures like the minimal clinically important difference (MCID).
The MFDS has yet to release the Council’s meeting minutes, which, under regulations, must be made public within one month of the meeting or within seven days after the application process concludes. An MFDS official said R Bio “did not submit definitive evidence” to overturn the prior ruling and that detailed review findings would only be disclosed with the minutes.
U.S. Market Entry Still Likely, FDA Decision Key
In contrast to Korea, U.S. regulators have shown support. In June 2025, the FDA authorized an Expanded Access Program for JointStem, allowing real-world use. Nature Cell is now preparing for FDA accelerated approval, selecting partner hospitals, and planning GMP facility construction at its “Biostar Stem Cell Campus” in September. The company aims to submit a Phase 3 application by year-end or early 2026, targeting accelerated approval in the first half of next year, which would allow commercial sales during the trial.
A company spokesperson said the sole reason given for Korea’s rejection was “lack of clinical significance” and that next steps in Korea will be decided after reviewing the meeting minutes. Regardless, CEO Jeong-chan Ra stated, “We will ensure JointStem receives U.S. approval through a fair and objective review. Based on sufficient scientific evidence, we will also work toward recognition as an advanced regenerative medicine technology in Korea.”
Nature Cell plans to strengthen cash flow through its regenerative medicine businesses in Japan and the U.S. and to attract strategic investment at home and abroad.
