Lilly Eyes ESMO 2025 Data to Bolster Case Following Fourth Rejection in Korea

Abemaciclib (brand name Verzenio), a CDK4/6 inhibitor, is preparing to reapply for reimbursement in early 온라인 바카라사이트 cancer following its third rejection. Eli Lilly plans to present new long-term survival data at the European Society for Medical Oncology (ESMO) Congress in October as part of its renewed push.
On July 23, the Health Insurance Review and Assessment Service (HIRA) announced that Korea’s 6th Cancer Disease Committee of 2025 had decided not to establish reimbursement criteria for Verzenio in combination with endocrine therapy. The proposed indication was for adult patients with hormone receptor-positive (HR+)/HER2-negative (HER2-) early breast cancer at high risk of recurrence and with lymph node involvement.
Early breast cancer refers to disease confined to the breast or nearby axillary lymph nodes, without distant metastasis. High-risk patients are defined as those with either (1) four or more positive axillary lymph nodes, or (2) one to three positive nodes with either histologic grade 3 tumors or tumor size ≥5 cm.
Although more than 95% of stage II–III breast cancer cases are potentially curable with surgery, 14–23% of patients still experience recurrence after adjuvant therapy. Among high-risk groups, recurrence rates are nearly doubled, reinforcing the case for post-surgical treatment options.
However, some experts believe Verzenio’s reimbursement prospects remain uncertain unless the drug can demonstrate a significant overall survival (OS) benefit. This is due to the already high long-term survival rates in early-stage breast cancer.
To address this, Lilly intends to submit upcoming 7-year OS results from the “MonarchE” trial—set to be presented at ESMO 2025—as new evidence to support its next reimbursement request. The company confirmed this strategy during a Verzenio media session held on July 16.
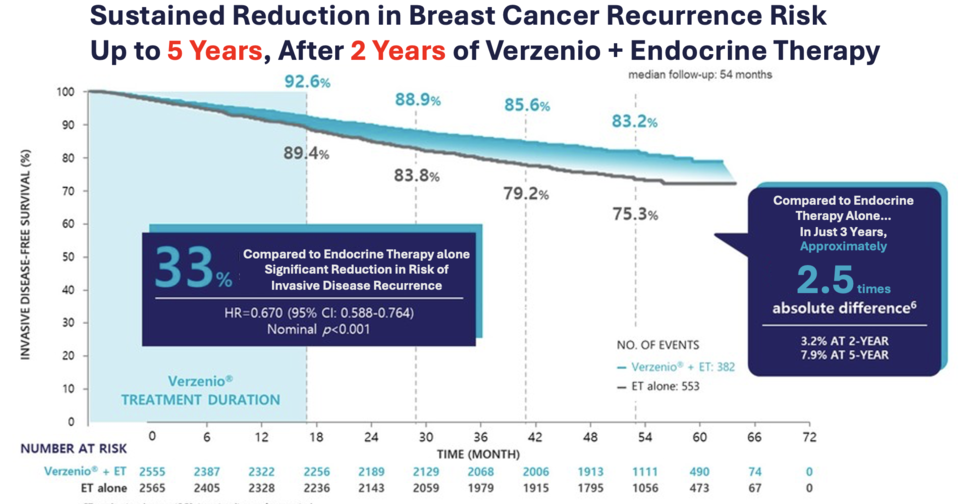
At the 2023 ESMO Congress, Lilly shared 5-year follow-up data from MonarchE showing Verzenio’s clinical benefit in high-risk early breast cancer. The 2-year combination of Verzenio and endocrine therapy reduced the risk of invasive disease or death by 33% (HR = 0.670, 95% CI: 0.588–0.764, nominal p < 0.001) and lowered the risk of distant recurrence or death by 33.5% (HR = 0.665, 95% CI: 0.577–0.765, nominal p < 0.001). However, the OS data remained immature, with median survival not yet reached.
A professor of oncology at a major tertiary hospital commented, “Most HR+/HER2- breast cancer patients in Korea are premenopausal. These women are often in the workforce or caring for families. Reimbursement decisions should consider not only OS improvements but also the broader social and economic value of helping them maintain daily life.”
Meanwhile, Novartis is closely watching Verzenio’s trajectory as it prepares for its own label expansion with Kisqali (ribociclib), another CDK4/6 inhibitor. Last year’s ESMO meeting featured 4-year data from the NATALEE trial, showing Kisqali improved invasive disease-free survival (IDFS) and distant recurrence-free survival (DRFS) compared to endocrine therapy alone.
Unlike Verzenio, Kisqali’s trial enrolled a broader patient population by not limiting eligibility to those with lymph node involvement. However, given Verzenio’s challenges despite its narrower target group, Kisqali may also face regulatory headwinds.
A representative from Eli Lilly Korea stated, “We cannot share specific details about future reimbursement strategies at this time. However, we remain committed to delivering innovative treatment options and expanding access for breast cancer patients in Korea.”
