Interview with HIMALAYA Study Lead Professors
STRIDE Proves 20% 5-Year 에볼루션 바카라 Rate, More Than Double That of Sorafenib
At the European Society for Medical Oncology (ESMO) Annual Congress 2024, five-year 에볼루션 바카라 data from the HIMALAYA trial, which evaluated the combination immunotherapy Imfinzi (durvalumab) and Imjudo (tremelimumab), known as the STRIDE regimen, was presented. Experts discussed the potential of this regimen as a new first-line treatment option for advanced hepatocellular carcinoma, potentially replacing VEGF inhibitor-based therapies.
The five-year follow-up data from the Phase III "HIMALAYA" trial, presented on September 16th, highlighted the efficacy of the STRIDE regimen for advanced or unresectable hepatocellular carcinoma. Conducted across 16 countries with 1,324 patients, the study evaluated the safety and efficacy of dual immunotherapy as a first-line treatment for adult liver cancer patients. The trial enrolled participants into four groups: Imfinzi monotherapy, two groups with varying doses of Imfinzi + tremelimumab, and a control group treated with sorafenib.
The results showed a five-year overall 에볼루션 바카라 (OS) rate of 19.6% in the STRIDE group, compared to 9.4% in the sorafenib group, with a median OS of 16.43 months for STRIDE, versus 13.77 months for sorafenib, reducing the risk of death by 24%. These findings mark a significant step forward in liver cancer treatment.
Hit News interviewed Professor Lorenza Rimassa, the global principal investigator of this study from Humanitas University and IRCCS Humanitas Research Hospital in Italy, and Professor Jeon Jae-hong from Bundang CHA Hospital’s Hematology and Oncology Department, who participated in the domestic trial. They discussed the significance of the phase III results and the clinical value of the STRIDE regimen.

You recently presented the five-year long-term follow-up results of the HIMALAYA study. What do these data mean for patients?

"The HIMALAYA trial data are the longest follow-up results from a Phase III study in unresectable liver cancer. The five-year OS rate for the STRIDE group reached nearly 20%, while the sorafenib group had an OS of around 9%. These results indicate that one in five patients receiving the STRIDE regimen can expect to survive five years, which is unprecedented in liver cancer treatment.
The safety profile was consistent, with 20 patients still on STRIDE therapy at the time of data analysis. These results suggest that tumor reduction, even when not achieving full remission, correlates with long-term survival, signaling a need to reconsider evaluation criteria in liver cancer treatment."
Why did you prioritize OS (Overall 에볼루션 바카라) over PFS (Progression-Free 에볼루션 바카라) as a primary goal?
"Improving overall survival is the most critical goal for patients with advanced liver cancer, who face a high risk of mortality. OS is the ultimate measure of treatment success. In immuno-oncology, progression-free survival (PFS) improvements don't always equate to longer life expectancy, as seen in the HIMALAYA results.
The survival curve for STRIDE continues to improve over time, widening the difference from the sorafenib group. This is a unique and groundbreaking outcome for liver cancer treatment."
The 에볼루션 바카라 regimen administers a high dose of Imjudo only once at the beginning. What is the reason for this, and what are its advantages?

"The initial high-dose administration is aimed at enhancing the response rate, a concept known as the ‘priming effect.’ To understand this, it's important to first explain how the cancer immunity cycle works, which is fundamental in the context of immunotherapy.
When antigens are released from cancer cells, dendritic cells (DCs) engulf these antigens and present them as peptides on their surface, allowing T-cells to recognize them. Once activated, these T-cells infiltrate the tumor and kill the cancer cells. The priming effect occurs when dendritic cells are further stimulated to give a stronger attack signal to T-cells. In the 에볼루션 바카라 regimen, Imjudo performs this priming role.
PD-L1 inhibitors like Imfinzi or atezolizumab are only effective when T-cells are already present around the tumor. As a result, when used alone, the response rate tends to be around 10-15%. However, administering Imfinzi after a high dose of Imjudo can lead to a much higher efficacy. This is why the dual immunotherapy approach holds significant value."
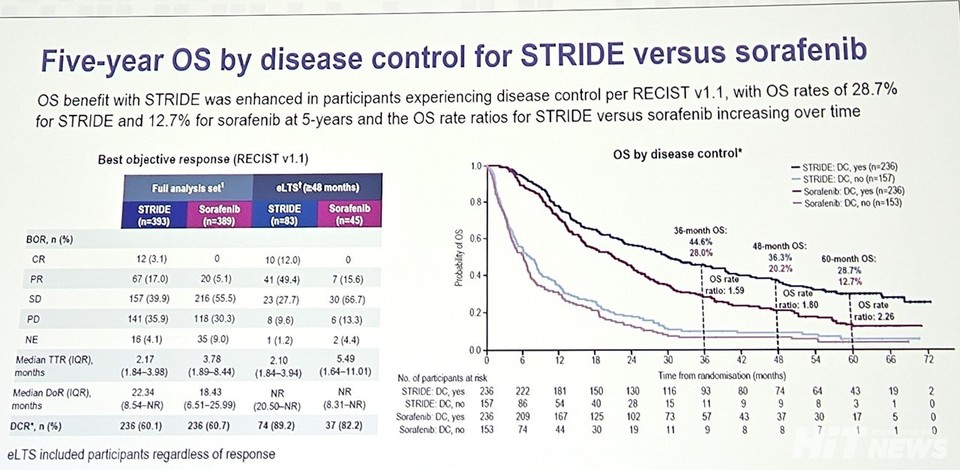
Subgroup analysis results related to 'disease control' were also released. Can you explain the findings and their significance?
"The key takeaway from this subgroup analysis is the relationship between tumor reduction and survival rates. The more significant the tumor reduction, the more pronounced the improvement in survival. This suggests that 'disease control,' which includes stable disease (SD) and objective response (OR), plays a crucial role in long-term survival. The five-year survival rates for these groups exceeded 28.7% and 20%, respectively.
Additionally, the analysis showed that patients with the largest tumor reductions—over 50%—received the greatest treatment benefits. Interestingly, even among patients with less than 30%, around 20% tumor reduction, approximately 25% achieved long-term survival, though this specific data was not presented at the ESMO conference."
What are the strengths of the 에볼루션 바카라 regimen as a first-line treatment for liver cancer?
"When choosing a treatment option, multiple factors must be considered, but in liver cancer, it is crucial to select a therapy that preserves liver function, which is different from other cancers.
In cancers like breast, stomach, or colon cancer, powerful drugs such as first-generation cytotoxic chemotherapy are needed to kill the tumor. However, these treatments are not suitable for liver cancer because, even if the tumor shrinks, liver function may deteriorate simultaneously, ultimately worsening the patient's overall condition.
In a recent study published by our team, in collaboration with European research teams, on the combination therapy of atezolizumab and bevacizumab, it was found that patients whose liver function deteriorated during treatment had a higher mortality rate than those whose cancer progressed. If cancer progresses during treatment, the risk of death increases ninefold, but if liver function worsens, the risk can increase up to twentyfold, underscoring the critical importance of maintaining liver function.
Balancing cancer control with liver function preservation is very challenging, but immunotherapy aligns well with this goal. Even though 에볼루션 바카라 uses a combination of two immunotherapies, the CTLA-4 inhibitor Imjudo is administered in a high dose only once, which keeps toxicity low. Additionally, long-term data show minimal decline in liver function. Clinical experts should take note of this key point.
Furthermore, current VEGF inhibitors like lenvatinib, cabozantinib, ramucirumab, and bevacizumab are associated with an increased risk of esophageal varices and gastrointestinal bleeding. Therefore, it is recommended that endoscopic evaluation of esophageal varices be conducted before use.
However, in real-world clinical practice, there is often a lack of human resources to perform endoscopic evaluations on every patient, making it practically difficult. While using VEGF inhibitors carries this risk, the use of immune checkpoint inhibitors such as PD-1 inhibitors, PD-L1 inhibitors, and CTLA-4 inhibitors alone can be done safely without such risks."
What areas of liver cancer treatment still need further development?
One major need in first-line treatment is the identification of biomarkers to guide personalized therapy. Second-line treatment options are also limited, with only one phase II trial currently underway, as most pharmaceutical efforts are focused on first-line therapies.
Expanding clinical trials to include patients with poorer conditions, like those with impaired liver function, is another priority. For instance, the 에볼루션 바카라 regimen is being evaluated in the phase IIIb 'SIERRA' trial for patients with more advanced liver issues.
New treatments, such as immunotherapy combinations and bispecific antibodies, are being explored, but there is a shortage of second-line options. Ideally, second-line therapies should go through phase III trials, but this is challenging due to shifting first-line treatment standards. Experts are now relying on real-world data to inform treatment recommendations and update guidelines. For example, lenvatinib was recently added as a second-line option after atezolizumab-bevacizumab therapy. However, the 에볼루션 바카라 regimen is currently only considered alongside sorafenib, highlighting the need for more second-line options.
Currently, the 에볼루션 바카라 regimen is not covered by insurance in Asia, except for Japan.
"Access to the STRIDE regimen remains limited due to insurance coverage issues, especially in Korea. Despite demonstrating a significant five-year survival benefit, the lack of coverage restricts patients' access to this important therapy. I believe that, given the data, STRIDE should be recognized similarly to the atezolizumab-bevacizumab combination therapy, which is already covered."
