FLAURA2 OS data intensifies competition with Leclaza-Rybrevant combo

AstraZeneca announced on July 21 that the final overall survival (OS) results from the Phase 3 FLAURA2 trial confirmed a statistically and clinically significant OS benefit for patients receiving 꽁 머니 바카라 (osimertinib) in combination with chemotherapy, compared to 꽁 머니 바카라 alone.
These findings signal a shift in the competitive landscape, as 꽁 머니 바카라-based regimens face growing pressure from rival combination therapies such as Leclaza (lazertinib) + Rybrevant (amivantamab), both targeting first-line treatment for locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC).
AstraZeneca added that the progression-free survival (PFS) advantage, previously demonstrated and a primary endpoint of the study, remained consistent in the final analysis.
FLAURA2 is a global Phase 3 study evaluating the efficacy of Tagrisso plus chemotherapy versus Tagrisso monotherapy in patients with EGFR mutation-positive, non-squamous NSCLC. Interim results presented at the 2023 IASLC World Conference on Lung Cancer showed a 38% reduction in the risk of disease progression or death with the combination (HR 0.62, 95% CI 0.49–0.79; p<0.001). Median PFS by blinded independent central review (BICR) was 29.4 months for the combination arm, versus 19.9 months for monotherapy.
Based on these data, Korea’s Ministry of Food and Drug Safety (MFDS) expanded Tagrisso’s approved indications in April 2024.

Dr. Pasi A. Jänne, principal investigator of the FLAURA2 trial and oncologist at Dana-Farber Cancer Institute, emphasized the importance of both extending survival and maintaining quality of life, particularly in long-term first-line treatments.
“This is especially critical where patients often remain active throughout therapy,” he said. “These results support both Tagrisso monotherapy and the combination regimen as standards of care.”
He also noted that the survival benefit was achieved despite the FLAURA2 trial allowing all subsequent therapies post-progression, making the OS improvement more robust.
Dr. Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, added, “These OS results further solidify Tagrisso as the backbone of EGFR-mutated lung cancer treatment, offering patients longer survival and better quality of life.”
AstraZeneca plans to present the data at upcoming global oncology conferences and submit to regulatory authorities worldwide. 꽁 머니 바카라-based combination therapy is currently approved in more than 80 countries.
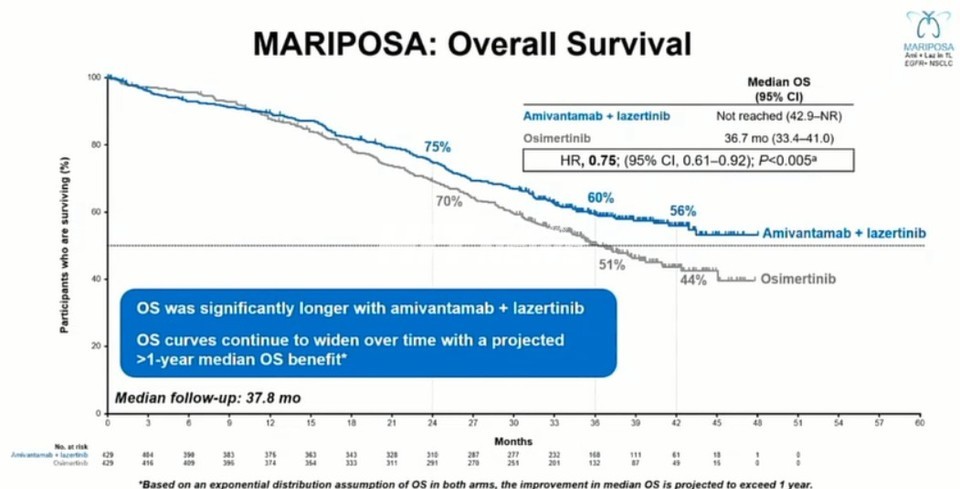
Meanwhile, attention is turning to the MARIPOSA-1 study, which directly compared Leclaza (lazertinib) + Rybrevant (amivantamab) with 꽁 머니 바카라 monotherapy in the same indication.
The final OS analysis—based on a median follow-up of 37.8 months—showed that the combination therapy reduced the risk of death by 25% versus Tagrisso (95% CI: 42.9–NE). Survival rates at 24, 36, and 42 months for the Rybrevant combination arm were 75%, 60%, and 56%, respectively. While the median OS has not yet been reached, investigators project a survival benefit of at least 12 months, potentially exceeding 50 months in total.
With key global lung cancer meetings approaching—such as the IASLC World Conference on Lung Cancer in September and ESMO Congress in October—upcoming data may further influence prescribing patterns, particularly in Korea.
