바카라사이트 소울카지노 Receives Approval for First Line Treatment of Biliary Tract and Cervical Cancers, Extending Reach to 29 Indications
The immune checkpoint inhibitor Pembrolizumab, known as Keytruda by MSD Korea, has broadened its indications to 29, reinforcing its position as a frontrunner in the "One Source Multi-Use" category. Recently approved by the Ministry of Food and Drug Safety (MFDS) on April 15th, Keytruda is now cleared for use as a first-line treatment for "locally advanced or metastatic biliary tract cancer" and "FIGO 2014 stage III~IVA cervical cancer" patients. In the case of biliary tract cancer, Keytruda can be administered alongside gemcitabine and cisplatin, while for cervical cancer, it complements chemoradiotherapy.
With this latest approval, 바카라사이트 소울카지노 establishes itself as the immune checkpoint inhibitor with the broadest spectrum of indications, a journey spanning just over 11 years since its initial endorsement for melanoma treatment in 2015. In comparison, its closest competitor, Opdivo (nivolumab), holds indications for approximately 20 conditions, illustrating 바카라사이트 소울카지노's significantly wider versatility within the realm of immune checkpoint inhibitors, typically limited to around 10 indications or fewer.
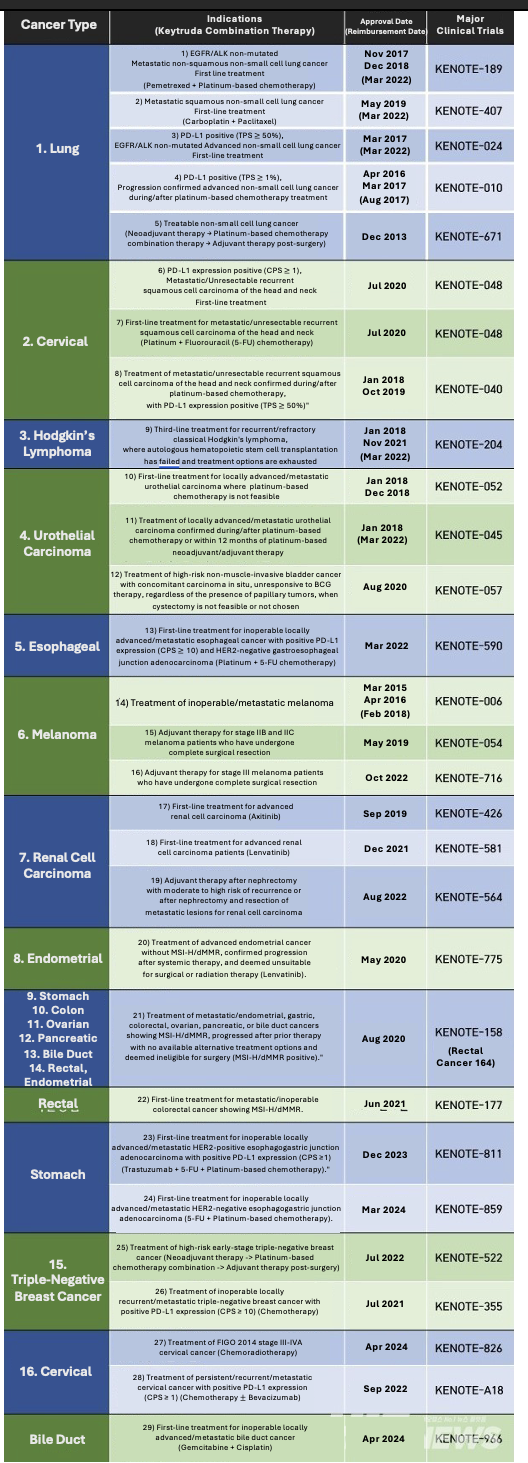
Keytruda’s South Korean approval indications and reimbursement and status (as of April 16th, 2024) / Graphic by reporter Jaeseon Hwang, Translated by Reporter Sodam Park
Additionally, 바카라사이트 소울카지노 qualifies for reimbursement across seven indications, targeting lung cancer, Hodgkin's lymphoma, urothelial carcinoma, melanoma, and others. The company has pursued reimbursement expansion for thirteen indications, including various breast, cervical, esophageal, bladder, and endometrial cancers, among others, initiating the process last June.
Moreover, MSD is actively engaged in numerous global phase III clinical trials, exploring combination therapies with antibody-drug conjugates (ADCs) and targeted therapy candidates sourced from Korean and international biotechs. Investment analysts foresee a continued expansion of 바카라사이트 소울카지노's indications.
Furthermore, the recent approval of Keytruda for first-line treatment indications for "surgically unresectable locally advanced or metastatic biliary tract cancer" and "FIGO (International Federation of Gynecology and Obstetrics) 2014 stage III~IVA cervical cancer" stems from global phase III clinical trials, namely, 'KEYNOTE-966' and 'KEYNOTE-A18'.
The KEYNOTE-966 study, involving 1,069 adult patients with biliary tract cancer, compared 바카라사이트 소울카지노 plus chemotherapy combination therapy with chemotherapy alone. The primary efficacy endpoint, overall survival (OS), demonstrated significant improvement in the 바카라사이트 소울카지노 combination therapy group. At a median follow-up of 25.6 months, a 17% reduction in the risk of death was observed (HR=0.83, 95% CI: 0.72-0.95, one-sided p=0.0034), with a median OS of 12.7 months (95% CI: 11.5-13.6), meeting primary efficacy endpoint criteria.
While the secondary efficacy endpoint, progression-free survival (PFS), did not reach statistical significance, the study revealed notable improvements in survival rates with 바카라사이트 소울카지노 combination therapy.
Additionally, the KEYNOTE-A18 study, encompassing 1,060 patients, assessed 바카라사이트 소울카지노 plus chemoradiotherapy (CRT) combination therapy against chemoradiotherapy alone in cervical cancer patients. The 바카라사이트 소울카지노 group exhibited superior progression-free survival (PFS) and overall survival (OS) rates, further supporting the efficacy of 바카라사이트 소울카지노 in first-line treatments.
관련바카라사이트 소울카지노
- 원소스 멀티유즈 끝판왕 '키트루다'… 적응증 29개로 확대
- [허가/임상] MSD, 'TROP-2 ADC+키트루다' 유방·폐암 3상 승인
- [허가/임상] MSD, TROP-2 ADC 활용 폐∙위암 임상 3상 승인
- '키트루다+항암화학' 병용, HER2 음성 위암 1차 치료 허가
- "키트루다, HER2 양성위암 1차 치료제로... 13년만의 새 옵션"
- 키트루다, 급여확대 신청 13개 적응증 중 일부 '재논의' 결정
- 핫한 바이오마커 시장... 탐스로신 불순물에 발목 잡힌 제조사
- '키트루다' 또 한번 급여확대 나서...13개 적응증 급여신청
- 담도암 1차 치료 진입한 '키트루다', 격차 벌리려는 '임핀지'
- "15개 적응증 급여확대 어렵네" 키트루다, 또 재논의
- "키트루다+CRT 병용, 25년만 등장 '조기 자궁경부암' 치료 옵션"
